
Delving into Angstrom-scale Absorption
Molecular sieve refers to a type of porous material with precise and uniform pore sizes on the angstrom scale, allowing for selective adsorption or separation of molecules based on their size and shape. An angstrom, symbolized as "Å," is a unit of length commonly used in scientific disciplines, particularly in the field of atomic and molecular physics. It is named after the Swedish physicist Anders Jonas Ångström. One angstrom is equal to 0.1 nanometers (nm). To provide some context, the typical size of an atom is in the range of 0.1 to 0.5 nanometers, which is equivalent to 1 to 5 angstroms. It is worth noting that the angstrom scale is often used to describe atomic distances, bond lengths, and molecular dimensions.
How Does the Angstrom Measure Up?
Angstrom to Human Hair Conversion
It would take approximately 1,000,000 angstroms (Å) lined up side by side to span the diameter of a human hair.
1 Angstrom to Millimeters Conversion
1 angstrom (Å) is equal to 0.0001 millimeters (mm).
1 Angstrom to Inches Conversion
1 angstrom (Å) is approximately equal to 0.0000000393700787 inches (in).
1 Angstrom to Meters Conversion
1 angstrom (Å) is equal to 0.0000000001 meters (m).
Absorption vs Adsorption
While the term "absorb" is often used informally to describe the process of molecular sieves removing or reducing moisture content, it is more accurate to say that molecular sieves adsorb moisture. Adsorption refers to the adhesion of molecules to the surface of a material, whereas absorption implies the penetration and incorporation of molecules within the substance itself. Therefore, it is more precise to state that molecular sieves adsorb or trap moisture, as they have the ability to selectively capture and retain water molecules within their porous structure.
Absorption
Absorption refers to the process in which one substance is taken in and incorporated into another substance at a molecular level. The absorbed substance becomes fully dissolved or distributed within the absorbing material. It involves the penetration and diffusion of molecules into the interior of the material. A common example is a sponge absorbing water, where water molecules permeate the sponge's structure.
Adsorption
Adsorption is the process in which molecules adhere to the surface of a material, forming a thin film or layer. The adsorbed molecules remain on the surface without being fully incorporated or dissolved into the material itself. Adsorption occurs due to attractive forces between the molecules and the surface of the material. An example is activated carbon adsorbing pollutants from air or water, where the pollutants adhere to the carbon's surface.
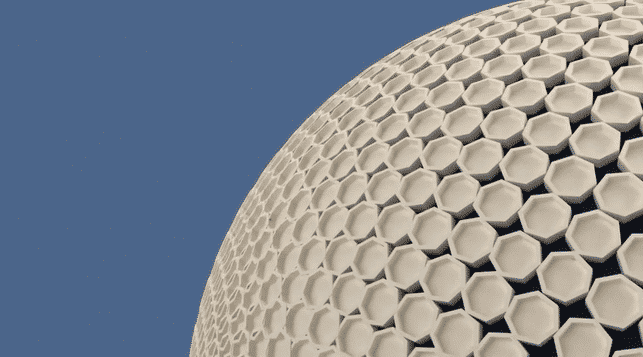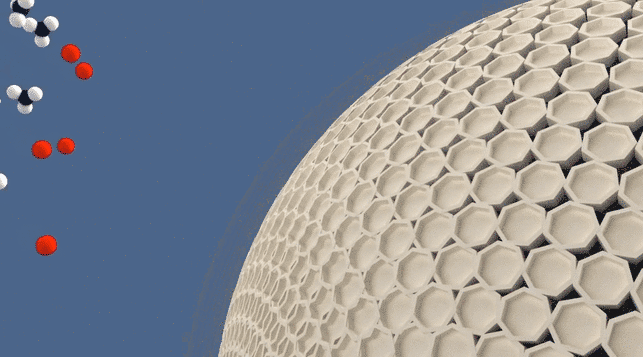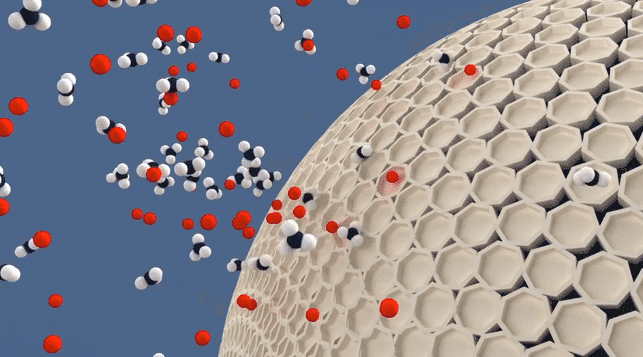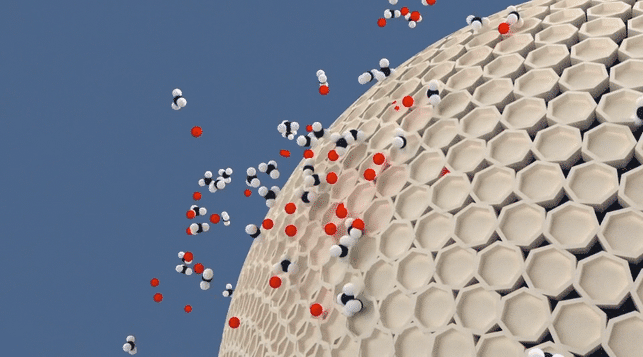
Molecular Sieve Animation
